™
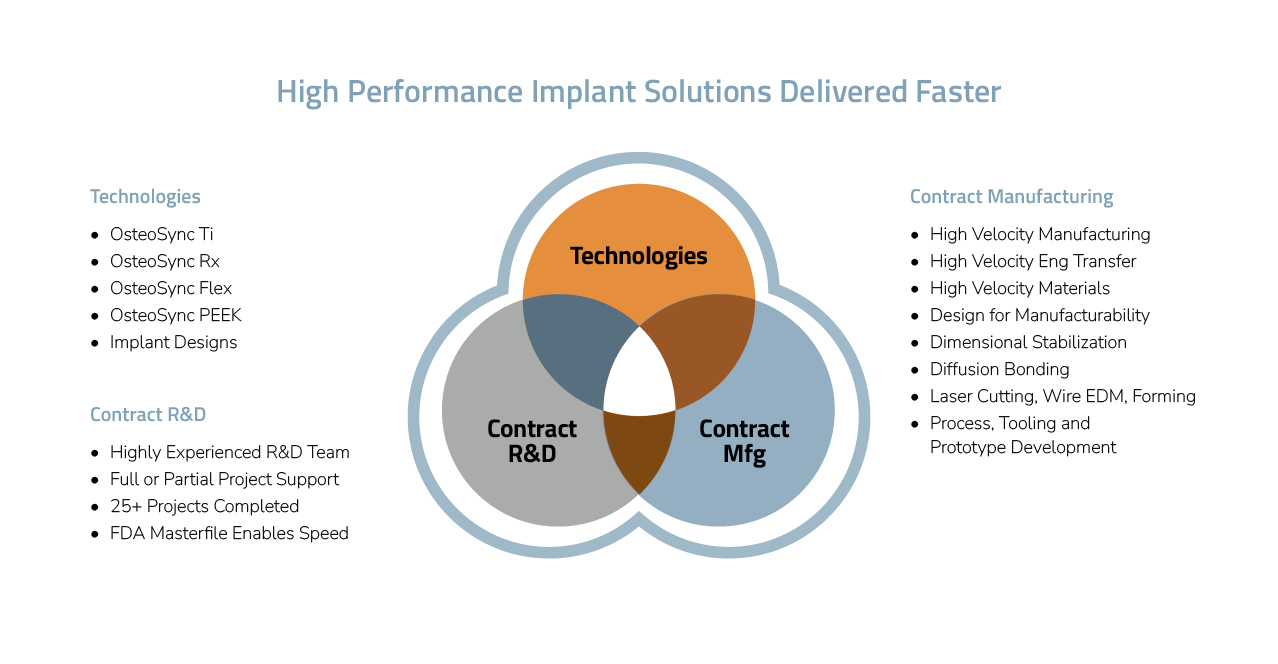
Orthopedic implant companies seeking a competitive advantage will find a trusted partner in SITES Medical. With a suite of innovative orthopedic technologies tailored to meet your needs, including those of your patients, we can bolster your success in today’s market. Our rapid product concept-to-launch process, backed by our extensive experience and unique R&D and manufacturing capabilities, is designed to support you every step of the way.
At SITES Medical, we focus on developing and de-risking innovative orthopedic technologies that enhance your product offerings. We collaborate with you to seamlessly integrate them into your product lines and bring them to market.
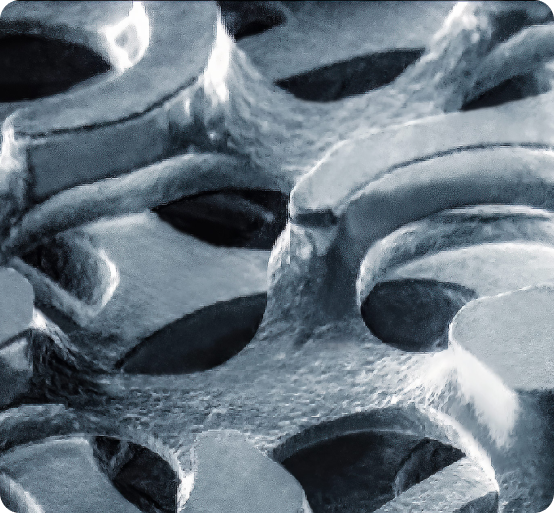
Our featured platform technology is OsteoSync Ti, a porous ingrowth material trusted by our OEM partners and marketed under various brand names. Additionally, we offer a range of technologies to meet your needs, including:

For orthopedic OEMs, SITES Medical provides comprehensive concept-to-launch R&D services, including regulatory filings. We provide a one-stop shopping approach, handling the entire process or specific parts, and can collaborate with your clinical team or help you build one using our extensive network. While our R&D services typically focus on projects involving our technologies, we’re open to considering other projects and can engage third-party R&D companies to support your needs.
We leverage our extensive experience and state-of-the-art equipment to provide full-service manufacturing capabilities that reduce costs and ensure the highest quality products.

At SITES Medical, we excel in several markets to meet your specific needs:
Orthopedic
(Hips, Knees, Extremities, Trauma, Sports)
Spine
(Interbody Fusion Devices, Disc Replacements, VBRs, Pedicle Screws, Plates)
Dental
(Implants, Reconstructive Meshes)
Other
(Vet, CMF, Cochlear)
Our deep expertise in these areas ensures that we provide you with the most advanced and effective solutions.
Nanovis, Inc. announced the acquisition of certain of its nano surface technology assets and certain of its rights to intellectual property assets related to Sites Medical’s OsteoSyncTMTitanium and related polymer molding technologies (used in fixed height PEEK/OsteoSync interbodies) by Medtronic for use in the development of Medtronic’s next generation PEEK interbody fusion devices.
Market acceptance of OsteoSync Ti continues to grow as we mark another key milestone for the technology – over 250,000 devices implanted! Our OEM partners appreciate OsteoSync Ti for its best-in-class bone ingrowth, high initial stability, ability to attach to various substrates (including Co-Cr and Ti), and compatibility with different coating technologies.
Microport is excited to announce the newest addition to the Evolution® Revision Knee family – Evolution® Tibial Cones. Designed to tackle the challenges of bone loss and poor bone quality encountered in complex revision knee surgeries, these versatile cones are made from OsteoSync® Ti and offer surgeons a reliable, efficient solution to improve patient outcomes.
SITES Medical in growth mode. In response to significantly increased demand for our OsteoSync Ti porous ingrowth technology, we’ve added six new, state-of-the-art wire EDM machines which are used in the manufacturing process.
Another milestone for OsteoSync Ti: Over 200,000 devices implanted. Our OEM partners have their own tradenames for it, but they all appreciate OsteoSync Ti for its best-in-class bone ingrowth, high initial stability and the ability to attach to various substrates, including Co-Cr and Ti.
SITES Medical celebrates its 15th anniversary. From humble beginnings and a focus on a singular technology, we have expanded our offering of platform technologies and added contract R&D and manufacturing services to provide a rapid product concept-to-launch process to support our OEM customers.
Spineology Inc., an innovator in anatomy-conserving surgery, is excited to launch the Duo Ti Expandable Interbody Fusion Procedure, which combines Spineology’s proprietary mesh technology with OsteoSync porous titanium to deliver a large, anatomy conforming implant via anatomy-conserving lateral decubitus and prone approaches.