
Full-Service Manufacturing Capabilities
At SITES Medical, we offer full-service manufacturing capabilities to help you deliver the highest quality products while reducing costs. Utilizing state-of-the-art equipment and deep production experience, we specialize in processes and products involving our advanced technologies, while our sister company, Mach Medical, provides full-service contract manufacturing for a wider variety of implants. Both SITES and Mach Medical are ISO 13485 certified, ensuring excellence in every project.
Specific Manufacturing Capabilities
- High Velocity Materials*
- Dimensional Stabilization*
- Diffusion Bonding
- Laser Cutting
- Wire EDM
- Forming
- Molding (Tool Design, Process Development, Production)
- 5-Axis Milling, Turning, Blast, Polish, Clean/Upgrade via Mach Medical
- Sterile Packaging and other Outside Processing via Partner Companies
* Are proprietary to SITES and bring unique benefits including reduced time to market and reduced cost.

*High Velocity Materials
For orthopedic products requiring castings or forgings, traditional near net shape development processes can take over 12 months. To avoid this, SITES Medical engineered a set of oversized, off-the-shelf castings and forgings that can accommodate various OEM designs and sizes. This approach allows us to quickly machine an implant to size and adapt to implant design changes without the need to re-design castings or forgings, thereby reducing time to market. Our OsteoSync Ti cementless knee femorals, made from high velocity materials, benefit from tighter tolerances and a more consistent press fit, thanks to machined inside box geometry and dimensional uniformity.
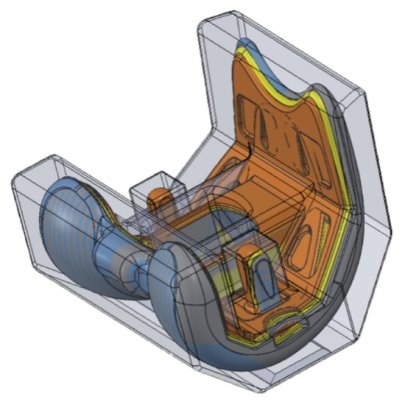
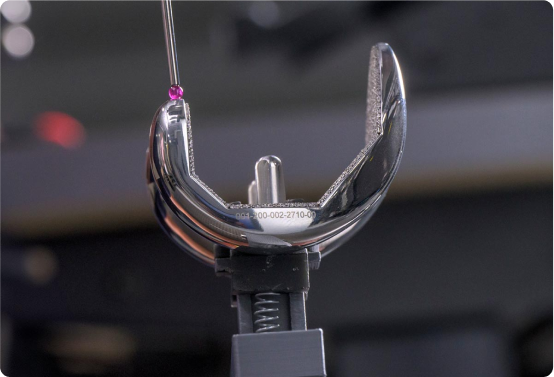
*Dimensional stabilization
During standard manufacturing of CoCr components, dimensional movement that occurs requires frequent inspections and adjustments to maintain precise dimensions. SITES Medical has developed a proprietary stabilization process that minimizes dimensional movement to a fraction of allowable tolerance across the complete part geometry. This innovation increases factory throughput and enables High Velocity Single-Piece Flow Manufacturing—a technology developed in collaboration with our sister company, Mach Medical. This approach ensures products can be manufactured with a lead time of just 3 weeks and in order quantities as low as one unit, in a highly cost-effective manner.
Let us help you achieve operational excellence and bring your innovations to life. Contact us today to learn how our comprehensive manufacturing services can support your needs and ensure the highest quality outcomes with your products.

